Researchers have recently uncovered a role for the kappa opioid receptor in mediating cold allodynia – an increased sensitivity to cold temperature.
Manish K. Madasu and Priyanka Chilukuri
Cold allodynia refers to pain caused by cold temperatures that are not normally perceived as painful and it is a hallmark of neuropathic pain disorders. Indeed, several disorders such as multiple sclerosis, fibromyalgia, complex regional pain syndrome, and chemotherapy-induced peripheral neuropathy can cause an abnormal and exaggerated sensitivity to touching or eating cold items. Despite the challenges faced by these patients, little is known about the molecular mechanisms underlying cold allodynia.
Researchers in the laboratory of Ream Al-Hasani, PhD, Associate Professor of Anesthesiology, have recently established a causal role for kappa opioid receptor signaling in increased sensitivity to cold temperatures in rodents. When they administered small molecules that activate the kappa opioid receptor, they found that mice demonstrated increased sensitivity to cold temperatures. Conversely, molecules that inhibit the kappa opioid receptor reduced behavioral outputs associated with cold allodynia. They published their preliminary findings on bioRxiv, a free online repository for work awaiting publication in a peer-reviewed scientific journal.
Molecules that activate the kappa (κ) opioid receptor often differ from those that affect the more commonly known and better understood mu (µ) opioid receptor. Drugs that activate the mu opioid receptor are known to produce painkilling and euphoric effects that have long been associated with their risk for misuse. Preliminary evidence suggests that molecules that activate the kappa opioid receptor have little to no potential for abuse because they have not been shown to produce the euphoria attributed to compounds that activate the mu opioid receptor.
Previous studies have demonstrated that activation of the kappa opioid receptor reduces pain caused by exposure to hot temperature, so researchers in the Al-Hasani lab had set out to determine whether the kappa opioid receptor was implicated in the mediation of noxious cold temperatures as well.
To establish a causal relationship between kappa opioid receptor activation and sensitivity to cold, the researchers exposed the paws of mice to different temperatures while treating the animals with molecules that activate or suppress kappa opioid receptor signaling. They measured cold sensitivity by quantifying the number of times mice jumped in a five-minute period. They found that when mice were given drugs that activated the kappa opioid receptor, they jumped more than control mice did, and mice that were given drugs that block the kappa opioid receptor. This indicates that activation of the kappa opioid receptor causes an increased sensitivity toward cold temperature exposure.
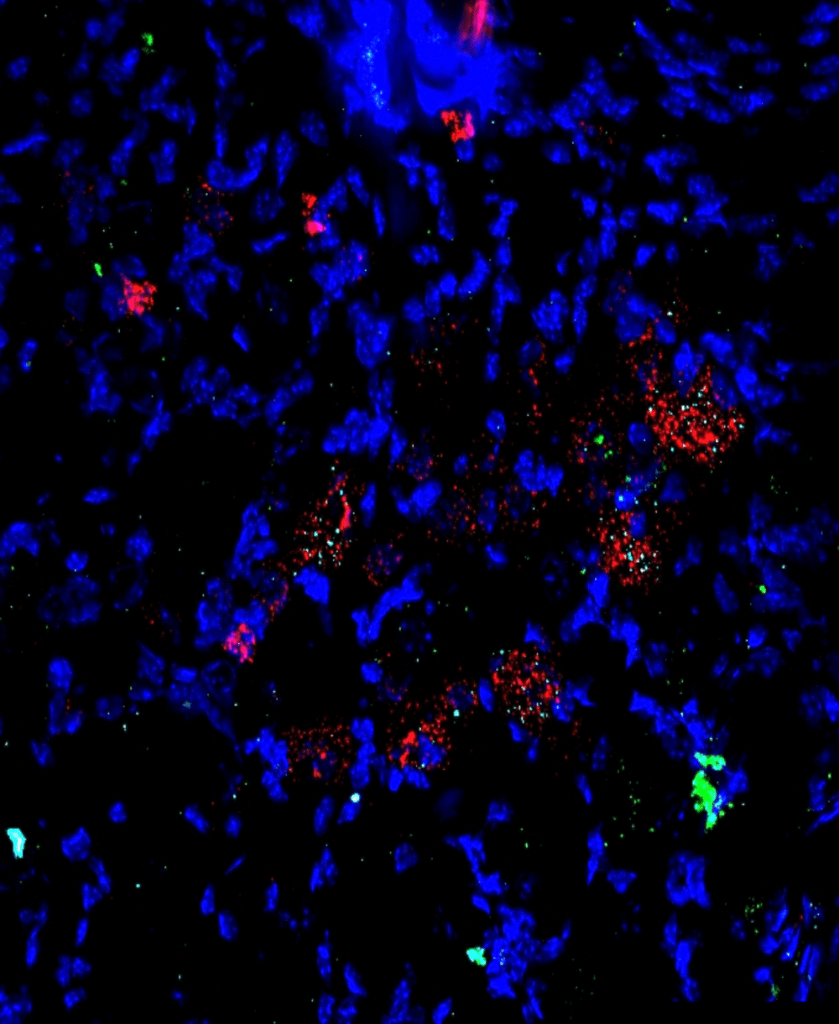
Then they looked within individual cells of the dorsal root ganglion, a bundle of neurons that relay sensory information from the body to the central nervous system. To determine the location of temperature-sensing channels within these cells, they had fluorescently stained them so that they could be further differentiated under a microscope. They found that kappa opioid receptors and channels that respond to cold temperatures – TRPM8 and TRPA1 – existed within the same cells.
The researchers then used calcium imaging to measure the activity of neurons in the dorsal root ganglion. This technique utilizes the tendency of neurons to intake calcium when firing an action potential. Therefore, the activity of this neuronal population in cell culture can be recorded to provide a readout of the kappa opioid receptor and TRPA1 activity using drugs that activate each of them. Cultured neurons from the dorsal root ganglion were first loaded with a fluorescent dye that binds to calcium. Neurons that are activated by these drugs will have more calcium activity than neurons that are not. They found that neurons from wild-type male mice were more active when given molecules that activated both kappa opioid receptor and the TRPA1 channel when compared to molecules that activate only one receptor type.
The researchers hypothesize that the kappa opioid receptor may induce cold hypersensitivity by amplifying TRPA1 signaling. The results of this study support the continued development of compounds that inhibit the kappa opioid receptor in the treatment of cold allodynia.
Kamden Kuklinski is a Neuroprep Postbacc Scholar in the lab of Ream Al-Hasani, PhD.