Inside Our Trainees’ Research: Labs & Publications
Publications
2025
(2025) Psychiatry and Neuroscience
Neuroprep Trainee: Laura Masso-Quinones
Abstract
The development and adoption of artificial intelligence (AI) provides moonshot opportunities to redefine how we generate treatments for neuropsychiatric disease. Despite the rapid advancement of AI across biomedical spheres, its implementation in drug discovery, proteomics, and neurobiology has been met with new and unexpected limitations. Historically, neuropharmacology research has used observational and invasive experimental approaches to identify novel therapeutics. Unfortunately, this classic approach suffers from laborious chemical synthesis and in vivo testing which ultimately leads to translational bottlenecks. With the implementation of AI, we are now able to expedite this early testing by modeling how a drug or protein complex may interact with a receptor of interest. By applying powerful, precision-based protein structure prediction tools, we can better tailor therapeutics and minimize undesired outcomes. Though promising, important caveats like predicting chirality of molecules, conformational changes upon binding, and determining downstream signaling elements remain critical roadblocks that functionally limit the efficacy of prediction software. This Perspective article will briefly discuss how AI-powered protein prediction software will impact drug development to transform neuropsychopharmacology research and therapeutics, while also providing insights into the limitations of these digital tools.
(2025) bioRxiv
Neuroprep Trainee: Alexa Toliver
Abstract
Despite the prevalence of chronic pain, the approval of novel, non-opioid therapeutics has been slow. A major translational challenge in analgesic development is the difference in gene expression and functional properties between human and rodent dorsal root ganglia (DRG) sensory neurons. Extensive work in rodents suggests that sensitization of nociceptors in the DRG is essential for the pathogenesis and persistence of pain; however, direct evidence demonstrating similar physiological sensitization in humans is limited. Here, we examine whether pain history is associated with nociceptor hyperexcitability in human DRG (hDRG). We identified three electrophysiologically distinct clusters (E-types) of hDRG neurons based on firing properties and membrane excitability. Combining electrophysiological recordings and single-cell RNA-sequencing (“Patch-seq”), we linked these E-types to specific transcriptionally defined nociceptor subpopulations. Comparing hDRG neurons from donors with and without evident pain history revealed cluster-specific, pain history-associated differences in hDRG excitability. Finally, we found that hDRG from donors with pain history express higher levels of transcripts encoding voltage-gated sodium channel 1.7 (NaV1.7) and 1.8 (NaV1.8) which specifically regulate nociceptor excitability. These findings suggest that donors with pain history exhibit distinct hDRG electrophysiological profiles compared to those without pain history and further validate NaV1.7 and 1.8 as targets for analgesic development.
(2025) bioRxiv
Neuroprep Trainee: Cabria Shelton
Abstract
Older adults often show improved emotional regulation with age, a phenomenon known as the aging paradox. This age-related increase in emotional regulation capacity is attributed to enhanced prefrontal cortex control over amygdala reactivity. However, because racial discrimination and economic disadvantage cause chronic stress, typical age-related neural associations may be altered in marginalized groups. Using task-functional MRI data from 8,711 UK Biobank participants aged 50-78, we investigated whether age-related associations in emotion-related brain function, specifically amygdala activation and vmPFC–amygdala connectivity, varied across racial and socioeconomic status (SES) groups. We found that older age was associated with decreased amygdala activation, which is consistent with improved emotional regulation. Yet, lower socioeconomic status was associated with increased amygdala activation, suggesting heightened stress-related reactivity. No significant age-related effects on vmPFC–amygdala connectivity were observed at the population level. Black participants showed a stronger age-related decline in functional connectivity compared to other racial groups. These findings call for more inclusive and diverse neuroimaging studies to better understand brain health across marginalized groups.
2024
(2025) bioRxiv
Neuroprep Trainee: Cabria Shelton
Summary
Neuroimaging data offers noninvasive insights into the structural and functional organization of the brain and is therefore commonly used to study the neuroimaging correlates of depression. To date, a substantial body of literature has suggested reduced size of subcortical regions and abnormal functional connectivity in frontal and default mode networks linked to depression. However, recent meta analyses have failed to identify significant converging correlates of depression across the literature such that a conclusive mapping of the neuroimaging correlates of depression remains elusive. Here we leveraged 23,417 participants across six datasets to comprehensively establish the neuroimaging correlates of depression. We found reductions in gray matter volume/ cortical surface area associated with depression in the frontal cortex, anterior cingulate, and insula, confirming prior studies showing the importance of prefrontal and default mode regions in depression. Our findings demonstrate multiple surprising results, including a lack of depression correlates in subcortical brain regions, significant depression correlates in somatomotor and visual regions, and limited functional connectivity findings. Overall, these results shed new light on key brain regions involved in the pathophysiology of depression, updating our understanding of the neuroimaging correlates of depression. We anticipate that these insights will inform further research into the role of sensorimotor and visual regions in depression and into the impact of heterogeneity on functional connectivity correlates of depression.
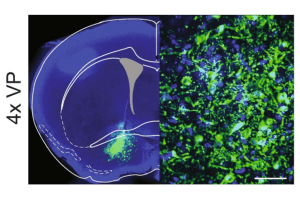
(2024) Science Advances
Neuroprep Trainee: Michelle Lynch
Abstract
The ventral pallidum (VP) has emerged as a critical node in the mesolimbic reward system. Modulating the VP can impact the subjective valuation of rewards, reward motivation, and reward seeking under conflict, making it an attractive target for clinical neuromodulation therapies that manage substance use disorders. To understand how to rationally modulate the VP, we need a better understanding of the electrophysiological properties of VP neurons and the molecular and biophysical determinants of these properties. Here, we used patch-clamp electrophysiology to characterize the intrinsic properties of glutamatergic VP (VPGlu) neurons and observed two distinct electrophysiological profiles: VPGlu neurons that undergo depolarization block in response to progressively increasing current injection amplitudes and those that were resistant to depolarization block. To explore the mechanisms that could contribute to these distinct profiles, we used targeted ribosome affinity purification to identify ion channel subunits and regulatory proteins by isolating actively transcribed mRNA selectively from VPGlu neurons. We then used this transcriptomic information to implement a Markov Chain Monte Carlo method to parameterize a large population of biophysically distinct multicompartment models of VPGlu neurons conforming to either subpopulation. Based on prior literature suggesting parvalbumin (PV) is expressed in a subset of VPGlu neurons, and that PV expression governs the firing properties of those neurons, we tested the hypothesis that PV expression accounted for differences in subgroups, by increasing the maximal firing frequency and conferring resistance to depolarization block. In contrast, our model determined that PV expression at physiological levels had no effect on maximum firing rate. However, supraphysiological expression levels of PV appeared to induce a depolarization block in previously depolarization block-resistant neuron models, suggesting that other intracellular calcium-binding proteins could play a role in determining the firing phenotype of VPGlu neurons. We corroborated this result with single-cell patch-clamp RT-PCR, which confirmed that PV expression did not distinguish the two electrophysiologically distinct subpopulations. Together, these findings establish that VPGlu neurons are composed of biophysically distinct subpopulations that have not been appreciated in prior studies interrogating the function of this population. With the advent of novel tools for cell-type specific pharmacology and targeted neurostimulation, this understanding will be critical for developing strategies to rationally modulate VPGlu cells to treat disorders characterized by maladaptive reward seeking.
(2024) Science Advances
Neuroprep Trainee: Michelle Lynch
Abstract
The ventral pallidum (VP) is critical for motivated behaviors. While contemporary work has begun to elucidate the functional diversity of VP neurons, the molecular heterogeneity underlying this functional diversity remains incompletely understood. We used single-nucleus RNA sequencing and in situ hybridization to define the transcriptional taxonomy of VP cell types in mice, macaques, and baboons. We found transcriptional conservation between all three species, within the broader neurochemical cell types. Unique dopaminoceptive and cholinergic subclusters were identified and conserved across both primate species but had no homolog in mice. This harmonized consensus VP cellular atlas will pave the way for understanding the structure and function of the VP and identified key neuropeptides, neurotransmitters, and neurotransmitter receptors that could be targeted within specific VP cell types for functional investigations.
Lab Features
As part of the Neuroprep Science Communication and Professional Skills workshop trainees write a news-style story that features a recent publication from their laboratory.